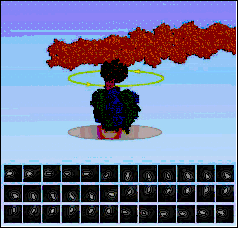
ATP-induced rotation of the g-subunit within the a3b3 barrel has been convincingly visualized by a single enzyme approach.
In these experiments, the a3b3g-fragment of the F1 portion from Bacillus PS3 was attached via three His-tags onto the glass surface of a coverslip, and, as an indicator, a fluorescent mm-long actin filament was connected to the g-subunit. The orientation of the actin filament with respect to the a3b3 hexamer was monitored by videomicroscopy. At very low ATP concentrations, rotation occurred in discrete 120° steps consistent with sequential ATP hydrolysis at the three b-subunits.