electrochemical pH control:
generating protons at the tip
1. redox reactions of mediators
a: KNO2 + H2O -> KNO3 + 2 H+ + 2 e-
b: p-bz-quinone + 2 H2O + 2e- -> p-bz-quinole + 2 OH-
2. principle of the chemical lens
-> buffer reduces diffusion field
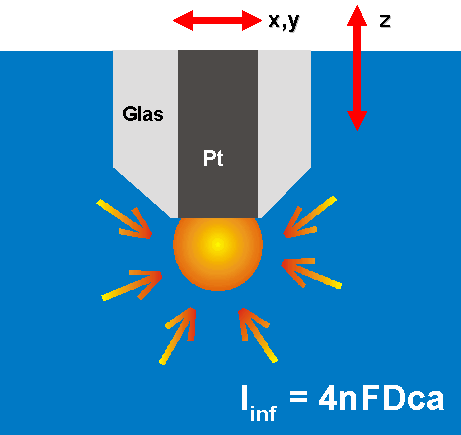
3. scanning electrochemical microscopy
SECM
-> piezo controlled nanometer movements
of a nm-sized platinum-electrode